Back to top
Regulatory Expertise & Quality Statement
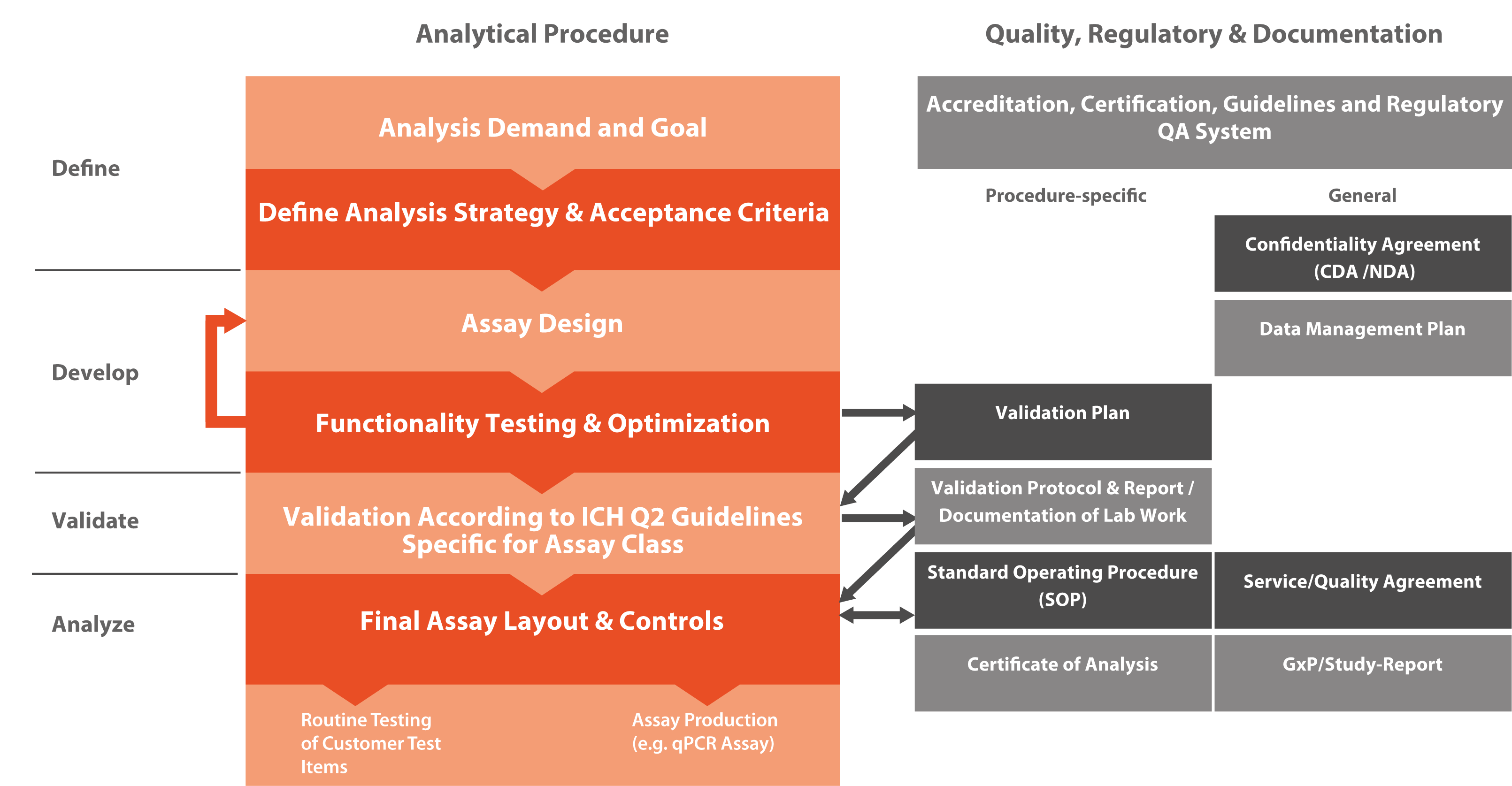
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has defined the standards for the development and registration of medicines. In VALIDATION OF ANALYTICAL PROCEDURES: TEXT AND METHODOLOGY (Q2R1), three major types of analytical procedures were defined: identification tests (for example a Class A assay showing that a particular personalized RNA vaccine has an identical sequence as expected), quantitative tests for specific DNA/RNA species (for example Class Bq assay to quantify genome edits in CRISPR/CAS9 test items), and limit test for the control of impurities (for example Class Bd assay demonstrating absence of contaminating RNA/DNA species in a test item).
Microsynth has experience in the validation of all three types of analytical procedures. All validation tests require high-quality reference material, either provided by the customer or developed by Microsynth. Identification tests are typically straightforward, while quantitative tests for impurities' content and limit tests for the control of impurities typically require a development phase to establish the relevant assays.
The validation will result in a report that defines the final assay, the details of the analytical procedure and the standard operating procedure (SOP) for the specific analysis. Important information about control samples and standards used in each analysis round are also provided. This information are instrumental to evaluate whether data of each testing round is valid by comparing the observed values against the expected values of the controls and standards.
Microsynth has experience in the validation of all three types of analytical procedures. All validation tests require high-quality reference material, either provided by the customer or developed by Microsynth. Identification tests are typically straightforward, while quantitative tests for impurities' content and limit tests for the control of impurities typically require a development phase to establish the relevant assays.
The validation will result in a report that defines the final assay, the details of the analytical procedure and the standard operating procedure (SOP) for the specific analysis. Important information about control samples and standards used in each analysis round are also provided. This information are instrumental to evaluate whether data of each testing round is valid by comparing the observed values against the expected values of the controls and standards.